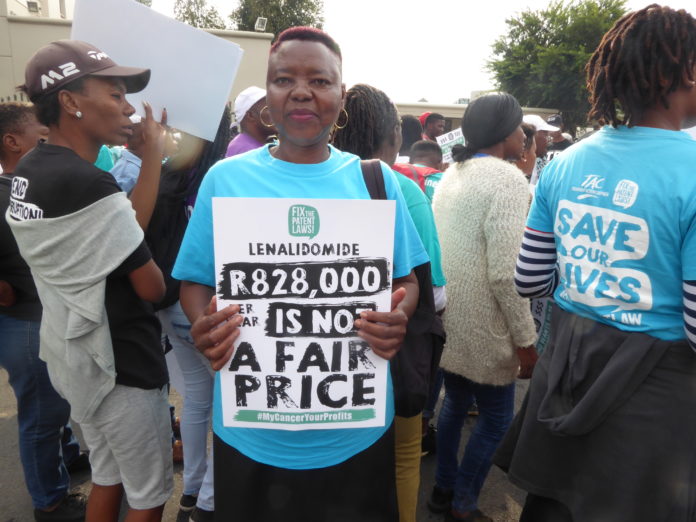“Medical innovation has little social value if most people cannot access its benefits…. this is a global human rights issue,” said Mariângela Simão, Assistant Director General at the World Health Organization (WHO), at the opening of the second WHO Fair Pricing Forum, co-hosted by South Africa, which took place 11-13 April 2019 in Johannesburg, South Africa.
The Forum brought together over 200 delegates from government, NGOs, academia and industry to discuss how to design a fairer pharmaceutical system where medicines are affordable to those in need and the development of effective new treatments is financed appropriately. Medicines Law & Policy was invited to present the TRIPS Flexibilities Database at the Forum; this database lists instances of the use of provisions in patent law to reduce prices on needed medicines, shedding light on how often they have been needed.
Calls for drug price and cost transparency grew louder at the second Forum, though they began at the first Fair Pricing Forum held in the Netherlands in 2017. High prices are defended by pharmaceutical companies as necessary to recoup costs of research and development (R&D) and incentivise further research, but with little clarity on how much R&D truly costs nor how medicines prices are set it is difficult to objectively determine what price is fair. Cases of exponential increases in the price of previously affordable drugs when market exclusivity was obtained were cited as examples that current prices seem to be more about profit than recouping costs.
Cancer took centre stage
The cost of cancer treatment took centre stage at the Forum, with the WHO’s report on the pricing of cancer medicines providing important background. The study found that the costs of R&D and production may bear little or no relationship to how pharmaceutical companies set prices of cancer medicines. Return on investment is more than generous with an average income of US$ 14.50 for every R&D dollar spent.
Cancer patients from South Africa gave powerful testimony at the Forum (and outside where activists were demonstrating and addressing the media) of the hardship caused by the high cost of the treatments.
One patient, who asked to remain anonymous, described his quest to gain access to the treatments he needs. Three years ago, he was diagnosed with multiple myeloma. His doctor prescribed lenalidomide, a derivative of thalidomide, which is sold under the brand name Revlimid by Celgene. In India, generic lenalidomide is available for R32,000 (US$ 2289) per patient per year. Until 2016, South African patients had access to generic lenalidomide from India under a section 21 authorisation that allows the sale and use of unregistered products. This authorisation was withdrawn once Celgene registered the product in the country. Celgene priced it at R882,000 (US$ 63,000) per patient per year. While the health system provides the drug, patients are struggling to pay the 20% co-payment. South Africa’s GDP per capita is US$ 6,160. According to the Cancer Alliance, South African patents block access to generic lenalidomide and this may last until 2028. Celgene told its shareholders earlier this year that it expects sales of Revlimid to be around US$ 10.8 billion in 2019. Bristol-Myers Squibb is currently in the process of taking over Celgene for US$ 74 billion.
There are similar issues with other cancer medicines. Trastuzumab (Herceptin), a WHO essential medicine for the treatment of HER2 positive breast cancer, costs R130,632 (US$ 9,295) in the public sector and R475,380 (US$ 33,827) in the private sector in South Africa. Biosimilar versions of trastuzumab are beginning to become available elsewhere in the world because the basic patent expired in 2016. But not in South Africa, where the company Roche continues to benefit from a market monopoly. The production cost of this same treatment is around US$ 240 for a one-year supply. Roche’s annual sales for the product is around US$ 7.5 billion.
Pembrolizumab (Keytruda) is a second-line treatment of non-small cell lung cancer and is priced at R180,000 (US$ 43,000). The Forum was told that to treat all in need would cost the South African health system R200 million (US$ 48 million).
These stories helped to set the scene and impress on participants to the Forum that there is no fairness in the system and that finding solutions to the problem of high drug pricing is urgent. Just as it was 20 years ago for HIV.
Pharma vs Nelson Mandela
In the past, South Africa has been the scene of some of the fiercest battles for access to medicines. Director General for Health, Precious Matsoso, recalled some of the histories at the opening ceremony. In 1998, at the height of the HIV crisis, 39 mostly multinational drug companies took the South African government to court over new provisions in its medicines law designed to accelerate access to lower-priced medicines. The companies claimed that the provisions were unconstitutional and in conflict with South Africa’s obligations under the TRIPS Agreement. Three years later, in 2001, the companies dropped the case after a global outcry and after it became clear that the legal text in question was based on draft legislation provided by the World Intellectual Property Organization (WIPO).
By then the case had become widely known as Pharma vs. Nelson Mandela and spurred global action on access to HIV medicines, strengthened flexibilities in patent law and made an industry in need of atonement agree to lower prices and later to offer patent licences to ensure low priced generic HIV medicines could be made available.
Twenty years ago, access to medicines was predominantly a problem for developing countries. “Today access to medicines has become a global issue”, said Simão.
Drug pricing a global issue
In Switzerland, the civil society organisation Public Eye requested the government for a compulsory licence on the patents related to the breast cancer medicine pertuzumab, sold by Roche under the brand name Perjeta. The US$ 100,000 price tag of Perjeta in Switzerland underscored the point that medicines pricing is a global issue. Similar demands have been made in other countries. Wilbert Bannenberg, chair of the Dutch Pharmaceutical Accountability Foundation described the case of a rare disease medicine, CDCA, used for the treatment of the metabolic illness cerebrotendinous xanthomatosis (CTX). For years, the product was available for € 300 per patient per year and prescribed off-label to treat CTX. After the drug company, Leadiant was granted exclusive rights to market the product in Europe for CTX in 2017, it increased the price to € 153,300 per treatment-year. Bannenberg’s presentation emphasised the need for competition authorities to take action against excessive drug prices. He further outlined his organisation’s plans to use the law (competition law, patent law, human rights law, civil law) to challenge unfair medicines pricing.
It may have been coincidence that shortly after the start of the Fair Pricing Forum, the Dutch Minister of Health Bruno Bruins announced his plans to reduce the exclusivity for orphan drugs from 10 to 5 years in the EUropean Union (EU) and to establish a commission to study the use of compulsory licensing to gain access to lower priced medicines in the Netherlands. A review of the pharmaceutical incentives in the EU is underway. Bruins also decried the lack of transparency in drug pricing and cost of R&D.
Dr Salmah Bahri, director of pharmaceutical services at the Malaysian ministry of health, described her country’s comprehensive approach to selecting and financing access to essential medicines. Malaysia does not shy away from using compulsory licensing when needed and has reduced the cost of hepatitis C treatments from US$ 72,000 to less than US$ 300 in the public sector by allowing generic import and supply of the product. They are also working with the Drugs for Neglected Diseases initiative (DNDi) on the development of a low-cost hepatitis C treatment.
Gaëlle Krikorian, from Médecins Sans Frontières (MSF), told the meeting about vaccine pricing challenges the organization deals with. MSF’s Access Campaign succeeded in persuading Pfizer and GlaxoSmithKline to significantly drop the price of the pneumococcal conjugate vaccine (PCV) for humanitarian organizations. Organisations working in emergency settings can now obtain the vaccine for US$ 9 per child under the ‘humanitarian mechanism’. It took seven years of negotiation to obtain the lower price. While this is good news for children in crisis settings others cannot benefit from this price. The list price of the vaccine is US$ 540 per child. In reality, prices vary but are high: France pays US$ 189, Lebanon US$ 243 and the price in local Greek pharmacies is US$168. MSF recently announced that it will use the “humanitarian mechanism’ to protect refugee children in Greece against pneumonia, the world’s ‘number one childhood killer’ according to MSF. Since 2009, Pfizer and GSK have earned $49.1 billion in sales from the pneumonia vaccine, said MSF.
Othman Mellouk from the International Treatment Preparedness Coalition (ITPC) also drew attention to the plight of people in middle-income countries. He mentioned the case of dolutegravir, an essential medicine for the treatment of HIV which will be available for US$ 75 per patient per year in low and middle-income countries. The patent license agreement reached by the Medicines Patent Pool with ViiV and the price deal brokered by the Clinton Health Access Initiative and partners exclude 39 countries, mostly upper middle-income countries. The World Bank defines lower middle-income economies as those with a GNI per capita between $1,006 and $3,955 and upper middle-income countries as those with a GNI per capita between US$3,956 and US$12,235. Above this figure, the country is considered high-income.
Transparency, transparency, transparency
The call for transparency emerged as the central theme of the meeting, in particular with regard to medicines pricing, production cost and expenditures on R&D. 64 civil society organisations published a statement before the meeting calling for greater medicines pricing and R&D cost transparency.
Andrew Hill presented data on the production cost of essential medicines that demonstrated most of them could be produced for a fraction of their current prices. For example, the price based on production cost for the US$ 30,000 per patient per year cancer drug imatinib can be as low as US$ 180 per patient per year. Insulin, which was discovered in 1923, cost US$ 1 per vial to make but is sold at US$ 240 per vial today. The global insulin market is valued at US$ 28 billion. Greater price transparency will help countries to understand the savings they can make by buying generics. The same call could be heard earlier this year at the Executive Board of the WHO. Since then, Italy has proposed a World Health Assembly resolution to improve the transparency of markets for drugs, vaccines and other health-related technologies. The resolution is expected to be discussed at the 72nd World Health Assembly that takes place from 20 – 28 May 2019.
The need for greater transparency in R&D cost was brought forward by the first Fair Pricing Forum, including the potential it has for designing targeted rewards for needed innovation.
Thomas Cueni, director general of the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) fiercely resisted the calls for transparency on medicines pricing. He warned greater transparency on medicine prices could backfire, and drive up drug costs in low and middle-income countries. This could, for example, be the case if high-income countries would refer to low prices available in low-income countries and demand the same lower prices or use it for reference pricing. Meeting participants seemed to agree with making provisions needed to allow for significant differential pricing. However, Suerie Moon from the Graduate Institute in Geneva pointed out that this would require more reasonable pricing in high-income countries as well. A fair price, she explained, is one that covers the cost made by the seller (including R&D, manufacturing and distribution, and fair profit) and assures affordability, value to the individual and the health system and security of supply to the buyers.
Jamie Love, director of Knowledge Ecology International, warned against using patient coverage as leverage in price negotiations. “Put the compulsory licensing of patents on the table, so that the monopoly is at risk, rather than the patient,” he said. He further outlined a proposal to finance the cost of R&D in a different manner. “Temporary monopoly is the primary incentive today, enforced by patents and a variety of regulatory monopolies. This is expensive and the primary reason why prices are high and access unequal,” Love said. By implementing models that de-link the cost of R&D from the price of medical products to finance drug development, high prices are no longer required to recoup R&D expenditures. He mentioned market entry awards and product prizes as two possible such models. He proposed a progressive implementation of de-linkage awards to gradually replace market exclusivities. A next step should be a feasibility study of the proposed new models. Such exploratory work would also benefit from greater transparency.
One successful example of an innovation initiative that works with a delinked model is the Drugs for Neglected Diseases Initiative (DNDi). The DNDi is open about its R&D outlays. “We need to be publicly accountable and be able to show the public’s return on the public investments we use to develop new treatment’ said Michelle Childs, head of policy advocacy at the DNDi.
The Medicines Patent Pool is another example of an organisation committed to transparency. The MPP publishes all its agreements in full on its website. The MPP initially focussed on HIV and later hepatitis C, but has expanded its mandate to negotiate licenses for all patented essential medicines. One company, with a significant cancer portfolio that was present at the Forum indicated that it is in talks with the MPP.
At the closing of the meeting, Fatima Suleman, a professor in pharmaceutical sciences at the University of KwaZulu-Natal and chair of the National Medicines Pricing Committee, noted the striking increase in research, available data and analysis to inform better policy making on medicines pricing and cost. Indeed, telling examples presented at the meeting included the WHO report on cancer drug pricing, the research on production cost of essential medicines by Andrew Hill, analysis on how to determine a fair medicine price and work on transparency by Suerie Moon, the TRIPS Flexibilities Database by Medicines Law & Policy, the patent opposition database, medicines patent analysis by I-Mak, the patent status information databases Medspal by the Medicines Patent Pool and Patinformed maintained by WIPO in collaboration with the IFPMA, and the drug research, development and pricing information database by Knowledge Ecology International.
What next?
The WHO announced it would set up working groups to further develop proposals put forward at the meeting before the next Fair Pricing Forum takes place in 2021. WHO will also launch an online public consultation in the coming weeks to collect suggestions for a definition of what constitutes a ‘fair price’. The next milestone will be the discussions on price and R&D cost transparency at the World Health Assembly this May of year.
The presentations made at the meeting will be available at the Fair Pricing Forum website.
Ellen ‘t Hoen, LLM PhD, is a lawyer and public health advocate with over 30 years of experience working on pharmaceutical and intellectual property policies.