In February 2024, the 13th World Trade Organization (WTO) Ministerial Conference will take place. WTO Members are expected to decide whether to extend the June 17 2022 Ministerial Decision on the TRIPS Agreement, which is currently aimed at increasing access to Covid-19 vaccines, to therapeutics and diagnostics. The original deadline for this decision was December of 2022.
The June 17 Ministerial Decision reinforces countries’ rights to use flexibilities contained in the WTO’s Trade-Related Aspects of Intellectual Property Rights (TRIPS) agreement. It also lifts, for Covid-19 vaccines, the export restrictions contained in TRIPS Article 31 (f) which determines that products produced under a compulsory licence must be predominantly for the domestic market. It further clarifies that a Member may authorise the use of the subject matter of a patent without the consent of the patent holder by any means available, whether the Member has rules for compulsory licensing (CL) or not. This is particularly useful for countries without procedures or with cumbersome procedures for issuing a CL. The decision also stipulates that TRIPS Article 39.3 (which protects against ‘unfair commercial use’ of test data needed to register a medicine) does not prevent the rapid marketing authorisation by medicines regulatory bodies. The Decision is currently limited to Covid-19 vaccines but would be more useful for other products, in particular therapeutics. According to the World Intellectual Property Organization (WIPO), 4,787 patent applications for Covid-19 therapeutics were filed between January 2020 and the end of September 2022. Further, it may be easier for effective use of the Decision to be applied to therapeutics and diagnostics as these can often be produced without access to additional know-how or technology.
In recent weeks, two documents have been published that should help to nudge Members towards extending the June 17 decision to therapeutics and diagnostics.
The first document is the long-awaited study carried out by the US International Trade Commission (USITC) upon request of the US Trade Representative Katherine Tai: COVID-19 Diagnostics and Therapeutics: Supply, Demand, and TRIPS Agreement Flexibilities (Inv. No. 332-596, USITC Publication 5469, October 2023). The second document is the TRIPS Council Chair’s report of the TRIPS Council’s Informal Thematic Session for External Stakeholders held at the WTO on 28 September 2023.
Neither report offers firm “yes or no” recommendations, but the data and evidence presented in both make a compelling case for extending the Decision to therapeutics and diagnostics.
The 493-page USITC report contains reports of discussions with various stakeholders and a comprehensive literature review. The report describes the disparity in access and availability of Covid-19 diagnostics and therapeutics: 80% of government procurement was by high-income countries, 14% by upper-middle-income countries, 5% by lower-middle-income countries and none by low-income countries. The report mentions that many factors affect access but highlights that high prices and the lack of price transparency appear detrimental to many countries seeking access.
From the literature review the USITC carried out it highlights that:
“From the available evidence, patent protection is generally found to be more beneficial to innovation in the health sector for developed countries and less so for developing countries. Patent protection is often found to result in higher prices for medicines, which decrease access, but patent protection can also have some counteracting effects, such as increases in international trade flows of pharmaceuticals and faster drug launches in markets, that help improve access. Researchers have found that compulsory licenses and the MPP are associated with increased generics and lower prices, and increased access to pharmaceuticals. Researchers have not studied the relationship between compulsory licenses and the MPP and access to COVID-19 diagnostics and therapeutics.”
The USITC encourages more research into the relationship between compulsory licences and the Medicines Patent Pool (MPP)and access to Covid-19 diagnostics and therapeutics.
The report goes on to acknowledge the advantages of CLs in terms of lowering prices and increasing access to needed products. The fact that a CL does not cover know-how, trade secrets and other forms of IP may be a barrier to effectively using the CL. This is especially true for new, complex technologies such as the mRNA Covid-19 vaccines. However, less complex molecules – and several Covid-19 therapeutics fall in that category – can be readily manufactured without access to additional knowledge. Another challenge listed is the lack of generic production capacity. Many countries that have used a CL in the past have relied on imports. Therefore, the waiver of TRIPS Article 31(f) export restrictions contained in the Ministerial Decision would be most welcome for use in therapeutics.
It also points out that in the past, countries that have used a CL have come under political and trade pressure from high-income countries such as the US and EU and the pharmaceutical industry. It also expresses the expectation that the 2022 Ministerial Decision can play a role in addressing this type of pressure. It says:
“The implementation of the 2022 Ministerial Decision has been highlighted as a potential means of reducing both this political pressure and potentially limiting retaliation from the pharmaceutical sector, as it reaffirms the right to issue a CL in a similar way to the Doha Declaration on the TRIPS Agreement and Public Health.”
Using, among other sources, ML&P’s TRIPS Flexibilities Database, the report does a good job of presenting the experience with CLs since the adoption of the Doha Declaration in 2001. See the illustration below.
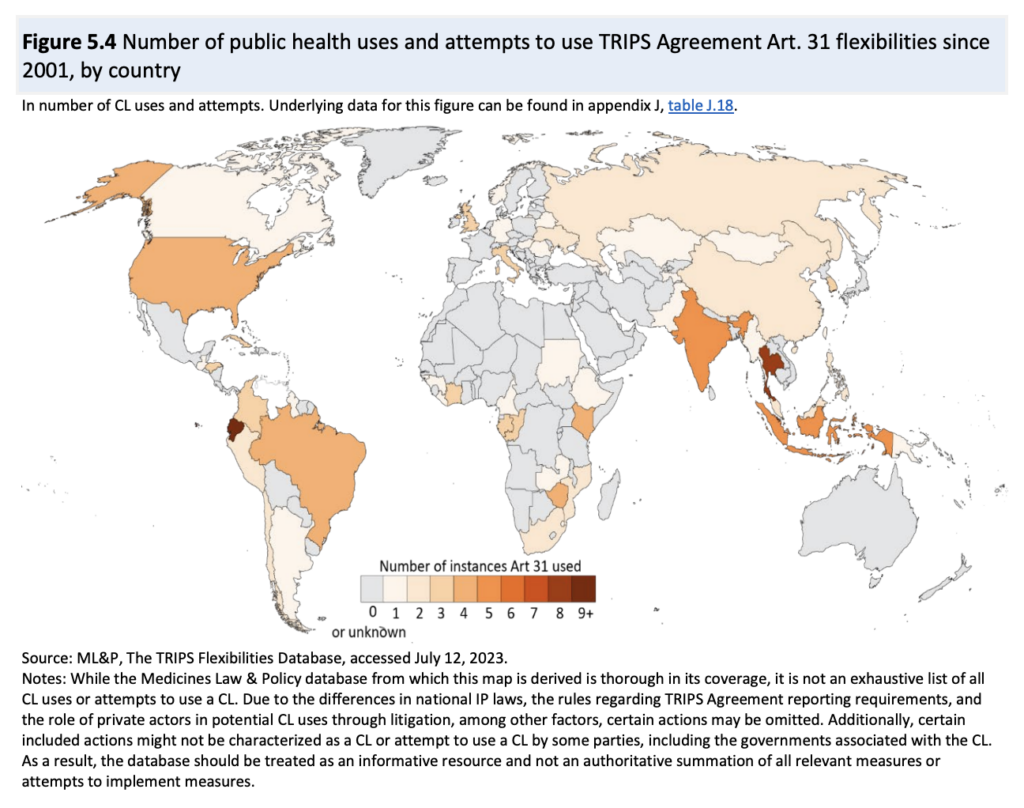
The report offers a comprehensive description of bilateral voluntary licences and Medicines Patent Pool licences. In both types of licences, the patent holder determines in which territories the products may be made available; in practice, this means that a significant number of middle-income countries are excluded from the agreements. Those excluded countries can, however, use compulsory licensing to gain access to the licensed products. MPP licences generally allow their sublicensees to supply to countries that have issued a compulsory licence. For details, see also the WHO/UNITAID country briefing. CL, therefore, remains an important option because tiered pricing by the originator does not offer the same advantages. The report says:
“In the case of COVID-19 therapeutics, tiered prices reportedly were multiples higher than the [generic] price negotiated by the Clinton Health Access Initiative.”
The USITC offers insights on the benefits of compulsory licensing, in particular for therapeutics that do not require know-how transfer. While the USITC carefully avoids taking a position on whether the June 17 2022 Decision should be extended to therapeutics and diagnostics, the evidence presented leads to the conclusion that extending it is sensible. This is how the USITC highlights the issue:
“In the available literature on the impact of CLs on pharmaceutical products, researchers have generally found that CLs are associated with decreased pharmaceutical prices in the countries that used CLs. The available research also associates CLs with increases in the number of people with access to patented products. There is some evidence that CLs encouraged innovation, where the literature has generally focused on the broader chemical industry.”
This conclusion that CLs are associated with lower drug pricing and increased access is supported by much of the evidence presented at the TRIPS Council’s Informal Thematic Session for External Stakeholders held at the WTO on 28 September 2023, and contained in the Chair’s report mentioned above.
A consensus emerged amongst most of the 22 speakers that licensing of IP is an important feature of the IP system to help enhance access to medical products. In particular, those medical products for which access to patents is sufficient to allow generic production. More complex technologies may require the transfer of additional know-how or technology.
The industry representatives stressed the role of IP in justifying R&D investments and urged the Council not to extend the decision to therapeutics and diagnostics. Offering the familiar talking point that doing so might undermine companies’ ability to invest in innovation.
This position is not supported by evidence as the USITC points out there is even some evidence that the use of CL stimulates innovation. Even in disease areas where the use of TRIPS flexibilities has been extensive, there has not been a negative effect on pharmaceutical innovation. This is not surprising because territories where TRIPS flexibilities are used, generally represent markets where the industry is absent with the products in question.
In conclusion, the two reports offer amble argumentation in favour of extending the June 17 Decision to therapeutics and diagnostics. It would, therefore, only be sensible for the WTO Members to do so. There is no reason for further delay.
Ellen ‘t Hoen, LLM PhD, is a lawyer and public health advocate with over 30 years of experience working on pharmaceutical and intellectual property policies.
