This week the European Parliament will vote on amendments to the Commission’s proposed regulation of the European Parliament and the Council on compulsory licensing for crisis management and amending Regulation (EC) 816/2006. The idea is that in times of crises, there should be efficient means to access needed countermeasures, including via compulsory licensing.
Alarmingly, some of the proposed amendments may make the regulation unworkable. In particular, the requirement proposed by the European Parliament’s Committee on Legal Affairs (JURI) that the Commission “identify in its decision the patent, patent application, supplementary protection certificate and utility model related to the crisis-relevant products, and the rights-holders of those intellectual property rights before granting the compulsory licence.” Further, the amendments stipulate that “Where the rights-holder or not all the rights-holders could be identified in a reasonable period of time, the Commission should not grant the Union compulsory licence.”
This sounds perhaps reasonable to an ill-informed reader but in reality, for many technologies, it would be difficult if not impossible to identify all rights holders in a timely manner.
The Commission itself points out that “a complete identification of all intellectual property rights and of their rights-holders may seriously undermine the efficient use of the Union compulsory licence to swiftly tackle the crisis or the emergency.”
The complexity of identifying patents and rights holders has various reasons: Often there are various rights holders and multiple patents (sometimes in the hundreds) for the same technology. Consider, for example, the patent landscape for HIV drug ritonavir, below, which shows the complex and growing web of different patents and rights holders related to one medicine. In a crisis, requiring such a mapping of interrelated technology would take too much time, and could cost lives. Sometimes rights holders themselves are in disagreement as to who holds the rights, as we have seen in the mRNA patent disputes.
In an emergency situation when governments decide to make use of a patent without the consent of the patent owner (which this regulation is about), the World Trade Organization TRIPS Agreement requires only that “the rights holder shall be notified as soon as reasonably practicable”. There is no obligation to identify all rights holders prior to the grant of a compulsory licence in an urgent situation. It is noteworthy also that United States law allows the government and its contractors to make use of patents without advance notice to patent holders.
The amendments risk paralysing the regulation’s effectiveness. Therefore, it is crucial that members of the European Parliament and the Council reject such amendments.
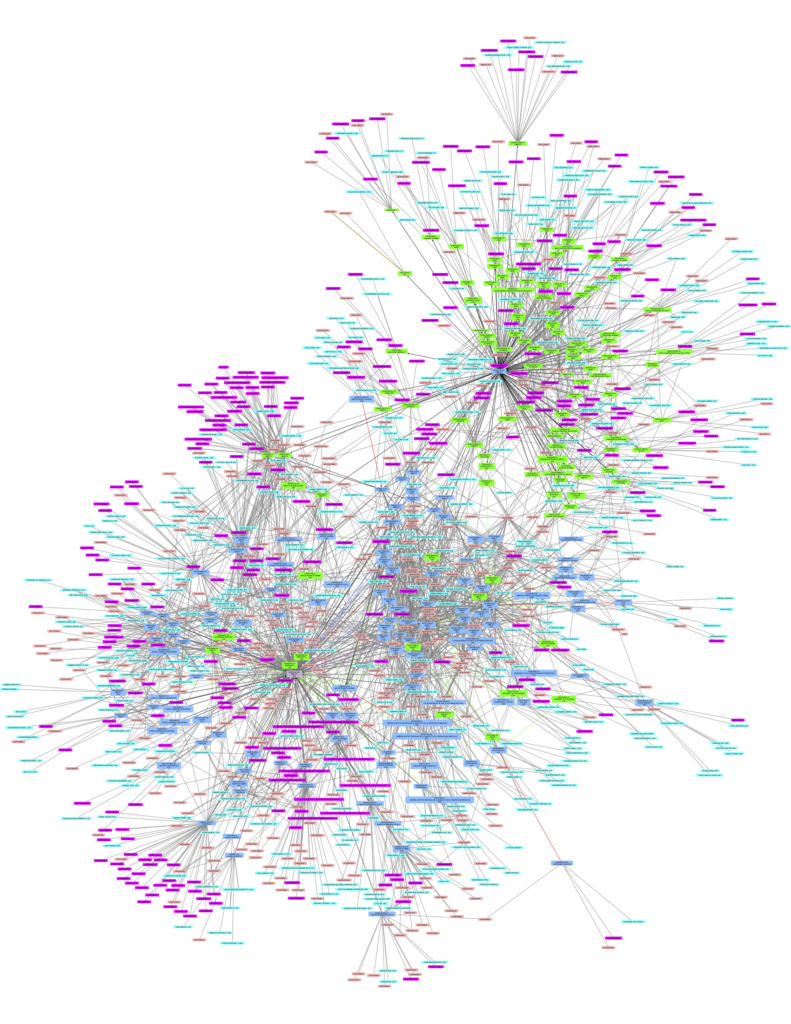
Citation map from the World Intellectual Property Organisation’s Patent Landscape of Ritonavir. Direct link to the infographic is here.
Ellen ‘t Hoen, LLM PhD, is a lawyer and public health advocate with over 30 years of experience working on pharmaceutical and intellectual property policies.
