The government of Malaysia has began to roll out treatment of people infected with the hepatitis C virus (HCV). Treatment will be a combination of antivirals (sofosbuvir and daclatasvir) from generic producers, including the Egyptian company Pharco, which has offered the curative 12 week HCV treatment for US$ 120. Egyptian companies are able to make generic HCV medicines because relevant patents on sofosbuvir and daclatasvir do not exist in Egypt. (For information about patent status of HCV medicines visit www.medspal.org)
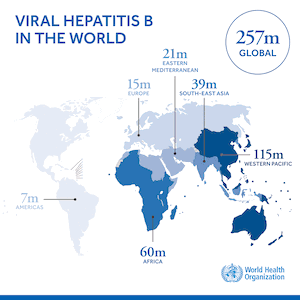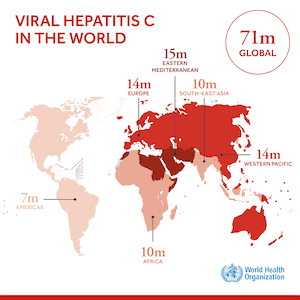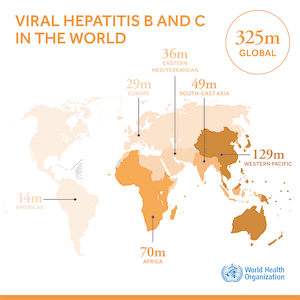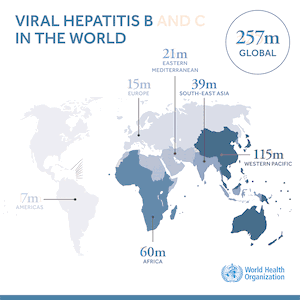
Malaysia, however, had granted patents on sofosbuvir. To enable purchase of low cost generic antivirals, on 20 September 2017 Malaysia issued a compulsory licence for sofosbuvir. As a result, the price of treatment has dropped from RM 50000 (US$ 13,000) to RM 1000 (US$ 258) and is expected to drop further to RM 500 (US$ 129) The decision to issue a compulsory licence to enable the purchase of low-priced generic products was central to the Malaysian government’s HCV treatment plan. With this move, Malaysia is responding to the call by the World Health Organization to step up efforts to eliminate hepatitis. An estimated 400,000 people infected with HCV live in Malaysia.
The patent holder, Gilead, responded to Malaysia’s compulsory licensing plans in August 2017 with an announcement that it would include Malaysia, Thailand, Ukraine and Belarus in the territory of its voluntary licence. The licence, however, only grants certain Indian companies the rights to supply in Malaysia. For details see here.
Compulsory licensing is one of the measures in the WTO TRIPS Agreement known as “TRIPS flexibilities” that can help in the procurement of low priced generic medicines when otherwise patents would form a barrier to doing so. The TRIPS Agreement contains other flexibilities. A very important one, only relevant to the least developed country Members (LDCs) of WTO, is the pharmaceutical transition, which allows 36 LDC Members to postpone the granting and/or enforcing of pharmaceutical product patents and clinical test date protection until at least 2033. The literature and media is riddled with statements that TRIPS flexibilities are not effective or have hardly been used. A recent study of 178 instances of presumed use of TRIPS flexibilities between 2001 and 2016 we published in the WHO Bulletin demonstrates that the reality is different. The study documents 100 compulsory licences, including government use licences, of medicines patents. Of those, 81 were executed, enabling the supply of generic medicines. In addition, we collected 40 cases of the use of the LDC pharmaceutical transition measure in procurement of medicines. And 26 LDCs procured medicines with reference to the paragraph 7 of the Doha Declaration on TRIPS and Public Health. The vast majority of instances of the use of TRIPS flexibilities we collected – 77.8% – was to gain access to low cost generic medicines for the treatment of HIV and related diseases.
The use of the flexibilities has been widespread and played a significant role in ensuring access to lower priced generic medicines in particular, for HIV. We conclude that the “study shows that integrating the use of TRIPS flexibilities in medicines procurement provides a legal and practical pathway to access lower cost generic versions of patented medicines.”
We can, however, not conclude that the use of TRIPS flexibilities is always an easy ride. For example, when India issued a compulsory licence in 2012 for a cancer medicine, it provoked an out-of-cycle review by the US Trade Representative. In 2016, Colombia came under pressure from Switzerland and a group of US senators for its plans to issue a compulsory licence for imatinib, a drug to treat leukaemia, with the US officials threatening the withdrawal of financial support for Colombia’s peace process. Measures are also being taken against Malaysia by the US. Professor Carlos Correa discusses some of these challenges in an editorial accompanying the WHO Bulletin study. It is also noteworthy, however, that the call by US politicians to use compulsory licensing domestically is getting louder. 18 members of the House of Representatives have asked the head of the US Health and Human Services to issue a compulsory licence to make treatment for hepatitis C affordable.
The TRIPS flexibilities can be powerful – but only as powerful as governments’ resolve to use them. Malaysia demonstrates how such resolve can and make strides towards the WHO goal of eliminating hepatitis C by 2030. Without deploying TRIPS flexibilities, they are often but words, providing false assurances that the TRIPS Agreement is flexible enough to be compatible with public health objectives.
The study was part of ‘t Hoen’s PhD thesis.
Ellen ‘t Hoen, LLM PhD, is a lawyer and public health advocate with over 30 years of experience working on pharmaceutical and intellectual property policies.