This month the European Commission published Innovative Payment Models for High-Cost Innovative Medicines, a report of the Expert Panel on effective ways of investing in Health (EXPH).
The report examines new ways of paying for new and costly medicines as a means to finding solutions for the ever-increasing prices of new pharmaceutical treatments. The panel does not shy away from exploring hot-button topics such as the need for greater transparency in medicines pricing, R&D and marketing cost, enlisting competition authorities to investigate drug pricing, ‘orphanisation’ of new medicines, revisiting the patent and market exclusivity as a cornerstone of innovation, developing alternative paths for innovation financing based on delinkage principles such as prize awards and the question how to ensure a ‘public return on public investment’. It further explores ways to bolster negotiating power of governments in price negotiations, including through the use of compulsory licensing of medicines patents.
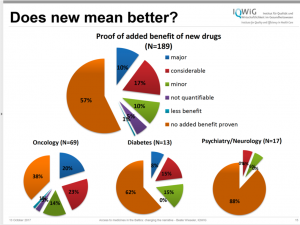
The panel makes recommendations for changes to the rules that currently govern how medicines are developed and brought to market, including the financing rules of the European Medicines Agency (EMA). The panel wants the EMA to be less beholden to the industry and suggests public funds to pay for the EMA’s work. Currently, the EMA largely depends on industry fees for its operations. Greater independence can help the agency to raise its standards for analysing new products. According to the Panel, today the small percentage of medicines with important clinical benefit is in contrast with the EMA’s drive to accelerate market access. Studies by the Insitute for Quality and Efficiency in Health Care show that overall 57% of new medicines have no proven added benefit. (Figure 1.)
Figure 1.
The high-ranking topics on the wish list of the Panel for a new payment model to address are:
- Addressing uncertainty about the true benefits of the new product,
- The desire to promote quick access of beneficial products to patients,
- Reward innovation,
- Promote innovation in neglected therapeutic areas,
- Maintain sustainability of health systems.
The Panel recognizes that only one type of payment model will not be able to address all these issues. But the current policy toolbox does not hold enough to formulate effective responses. Developing new approaches to innovation and payment models requires greater transparency and public engagement.
This is acknowledged by the Panel to some extent. It calls for more information exchange and dialogue between stakeholders and platforms to facilitate such dialogue. But it is unclear why the Panel limits participation in such dialogue platforms to governments and industry.
Many of the recommendations discussed in the Panel’s report are not new. They find their echo in the recommendations of the UN High-Level Panel on Access to Medicines and Innovation and the Lancet Commission on Essential Medicines Policies as well as numerous reports by the World Health Organization and national bodies (see for example the Dutch Health Council’s report on innovation and access. The OECD’s project on sustainable access to innovative therapies is also expected to publish its recommendations any time now. (Though word on the street is that the recommendations may be toned down under pressure from vested interests. Here is our contribution to the OECD consultation.)
What makes this expert report special is the fact that it comes at a time when the need to address high medicines prices in Europe has reached emergency levels. An EU wide approach to tackling high medicines pricing is still lacking, but EU member states increasingly cooperate in their quest to stand up to pharma’s price demands. There is an EU wide consensus that tackling pricing requires addressing the way we pay for innovation. In June 2016 following the Council Conclusions on Strengthening the Balance in the Pharmaceutical Systems in the EU and its Member States, the European Commission is reviewing current incentives for pharmaceutical innovation that affect medicines pricing. The results of that work should be published soon. The Expert Panel’s report should be an important contribution to European policymaking.
The industry called an earlier draft of the report “a missed opportunity to improve patient outcomes and support the sustainability of our healthcare systems” in a 48-page commentary published by EFPIA in December last year. EFPIA’s heavy-handed response which apparently delayed the publication of the report did not significantly alter the content.
Here is a summary of recommendations taken from the report.
- Greater price and cost transparency, including the acknowledgement that high prices (high costs to payers) may or may not have underlying high costs of R&D.
- Revisit the promotion of innovation through patent law and market exclusivity, as other mechanisms to promote and reward high-value innovations can and should be devised. This is particularly true when clear areas of neglected attention can be identified in a consensual way. The patent system is the current best option for decentralized innovation efforts when consumers are price sensitive, but not necessarily otherwise. This opens space to explore new models of promoting innovation that will encompass novel payment models which may or may not be associated with different rules in R&D funding (say, making use of prize-awarding mechanisms).
- Develop methodologies to measure the social value of pharmaceutical products and systematically use such methods, for instance in the context of Health Technology Assessment.
- Have an assessment of exercise of market power in each price negotiation, as insurance protection set by health systems reduces the role of consumer’s price sensitivity in limiting price increases of new products under patent protection.
- Set better rewards for higher therapeutic value added, so that innovation efforts are directed to the more relevant areas.
- Payment systems should evolve in the direction of paying for acquisition of a service (treatment) and not of a product (pill).
- Explore non-linear payment systems, including bundling, price-volume agreements, differentiation across geographies, and across indications, ensuring the conditions required for all parties to benefit.
- Create dialogue platforms involving all relevant stakeholders.
Ellen ‘t Hoen, LLM PhD, is a lawyer and public health advocate with over 30 years of experience working on pharmaceutical and intellectual property policies.
