This entire article is also available as a PDF by clicking here.
1. Introduction.
How relevant are concerns about intellectual property rights causing problems for scaling up production of Covid-19 related diagnostics, therapies and vaccines? Haven’t some important pharmaceutical companies announced that they won’t enforce their patents during the pandemic? Even if others do decide to enforce their patents, won’t the availability of compulsory patent licences solve the problem?
Patents are indeed a concern but they are not the only form of intellectual property right which is relevant in thinking about access to diagnostics, therapies and vaccines. A patent provides its owner with the right to stop others from, for example, making or using their invention. It is a ‘negative’ right. If the owner decides not to enforce their patent, however, or if a compulsory licence to the patent is granted, that does not necessarily mean that others positively can make or use the invention. There might be other intellectual property rights blocking the way. In fact, the patent owner may themselves own two other types of intellectual property right based on ‘undisclosed information’ which, leaving aside the patent, could still prevent others from making or using the invention.
This note starts with a ‘back to basics’ look at what information does and doesn’t have to be disclosed by a patent applicant and examines how undisclosed information (including ‘know-how’ and ‘test data’) is treated under the World Trade Organisation (WTO) Agreement on Trade Related Aspects of Intellectual Property (‘TRIPS Agreement’). It shows how three corresponding types of intellectual property rights – patents, know-how and ‘data exclusivity’ – form a ‘stack’ and how the ability or failure of third parties to adequately access all three elements of that stack (whether in a voluntary way, with the consent and collaboration of the rights holder, or in a non-voluntary way) will impact scaling up production of Covid-19 related diagnostics, therapies and vaccines. It concludes that trying to obtain access to the know-how element in non-voluntary cases will likely be the most challenging problem and looks at how that ‘know-how gap’ might begin to be bridged.
2. Background.
2.1 What must an applicant for a patent explain to the public?
Art. 29.1 TRIPS provides that:
“Members shall require that an applicant for a patent shall disclose the invention in a manner sufficiently clear and complete for the invention to be carried out by a person skilled in the art and may require the applicant to indicate the best mode for carrying out the invention known to the inventor at the filing date or, where priority is claimed, at the priority date of the application.”
An essential element of the ‘patent bargain’ between a patent applicant and the public is that the applicant must explain to the public (speaking to a competent worker in the field of the invention – a ‘person skilled in the art’) how to practically carry out the invention. If the invention relates to a product, this includes how to make and use the invention. It is this information, increasing the stock of public knowledge, which is supposed to be the quid pro quo for the time-limited exclusive rights which a patent confers.
Informed by different historical practice in different states, Art. 29.1 TRIPS permits a WTO Member (‘Member’) to choose between requiring an applicant to explain a way of carrying out the invention (even a poor way, so long as it is sufficient) and the best way (‘best mode’) known to the inventor. The distinction between the two choices has important practical consequences. If a best mode disclosure is not required and, for example, if a patent applicant knew two ways of carrying out the invention, a poor but sufficient way and a better way, they would be permitted to disclose only the poor way whilst keeping the details of the better way secret as ‘undisclosed information’. If a best mode disclosure is required, then the applicant must increase the scope of the disclosure to describe this better way.
In a spirited defence of best mode disclosure in the United States and elsewhere, Carlson, Przychodzen and Scamborava (2005) argue that:
“Absent the best mode disclosure obligation, the primary purpose of the patent system would be frustrated because the inventor would be permitted to retain the details of the invention as trade secrets while gaining the benefit of the patent monopoly. Such a result would allow inventors to effectively “have their cake and eat it too”…After the patent’s expiration, the inventor would be able to continue to maintain the “heart” of the invention as a trade secret…In short, without the best mode requirement, the entire foundation of the patent system is weakened, and the patent system itself is placed at risk.” (p. 93)
This being the case, it is perhaps surprising that a best mode disclosure requirement has not been adopted by all Members. For example, a sufficient disclosure rather than a best mode disclosure is all that is required in Europe (under Art. 83 of the European Patent Convention (EPC) (1973, as last amended 2000)).
2.2. When must an applicant for a patent disclose the best mode?
Even if a Member does decide to choose a best mode disclosure requirement, all that is required under Art. 29.1 TRIPS is the best mode known to the inventor at the filing (or priority) date. In many cases involving pharmaceutical inventions, it is likely that the inventor will be employed by a pharmaceutical company and (through the contract of employment) the pharmaceutical company will therefore be the patent applicant. The Art. 29.1 TRIPS requirement applies to the best mode known to the inventor rather than, in this case, that known to their pharmaceutical company employer. The pharmaceutical company may employ different teams, with different expertise, to make inventions and to develop and commercialise inventions that have been made.
Further, a patent application passes through a number of important administrative stages on its way from filing (at the filing date or, if priority is claimed, at the priority date, up to one year beforehand), through publication (typically eighteen months after the filing or priority date) and examination (by an examiner in a patent office) to eventual grant or refusal. The filing (or priority) date is therefore located right at the beginning of the patent application process.
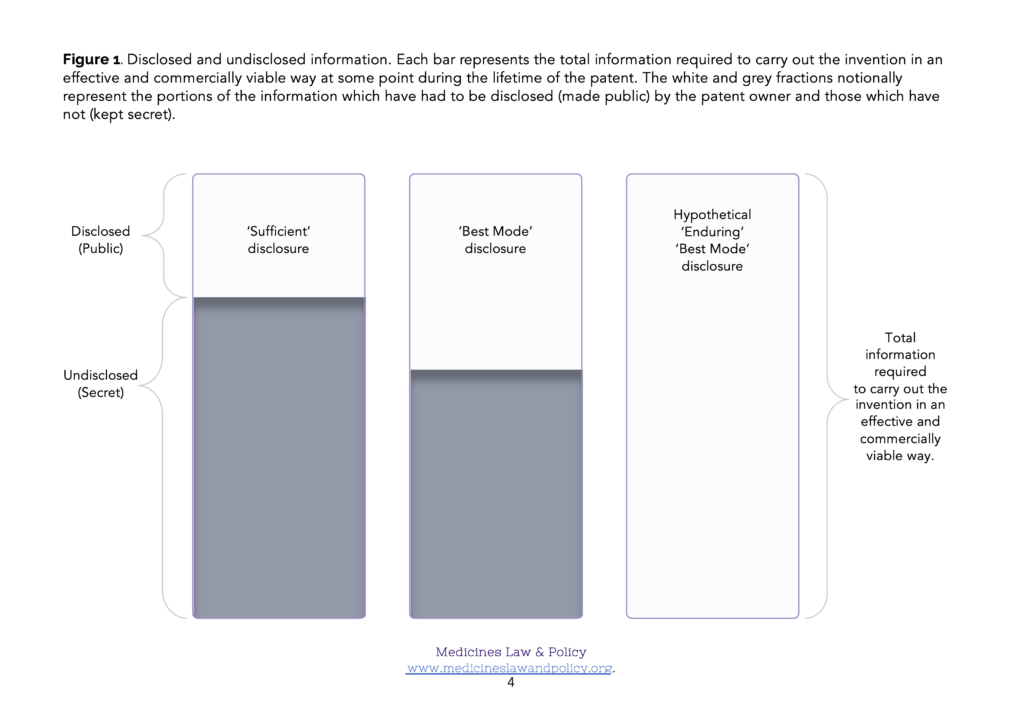
This means that if the patent applicant (pharmaceutical company) happens to find a much better way of carrying out the invention on the day after the filing date, they can keep it to themselves as ‘undisclosed information’. The same is true all the way through the patent application process to grant or refusal. Even more likely perhaps, in the case of an invention which was proving to be a success, if the patent owner (pharmaceutical company) finds a much better way of carrying out the invention during the years of patent lifetime following its grant, they can likewise keep it to themselves as undisclosed information. Given Carlson, Przychodzen and Scamborava (2005)’s strongly expressed arguments, it is sobering to note that this is so even if it is only with such an improved method that the invention can be carried out effectively and in a commercially viable fashion. Figure 1 illustrates such cases where (left hand column) a best mode disclosure was not required and (middle column) where a best mode disclosure was required.
There are, of course, good reasons for viewing the patent bargain as a ‘one-off’ transaction which is complete at the filing date. One is legal certainty. The scope of the exclusive rights which can be properly claimed in a patent are based on its description of the invention. If that description were permitted to change over time then the scope of the corresponding claims might change over time too. There are also, of course, good reasons for wanting to encourage patent owners to continue investing time and resources in improving their methods for carrying out their invention after the filing date. Nevertheless, again following in the sense of Carlson, Przychodzen and Scamborava (2005)’s arguments, if the public exchanges what may only be an early and bare snapshot of information about the best way (or at least a ‘sufficient way’) of carrying out an invention for twenty years of exclusive rights (potentially permitting very valuable monopoly market positions to be constructed and exploited) but the public then still fails to have access to an effective and commercially viable method of carrying it out, even after the patent has expired, that could easily look like the patent owner again ‘having their cake and eating it too’.
2.3. What is undisclosed information and how is it protected?
2.3.1. Undisclosed information.
In the above example, it might be that the improved method developed by the patent applicant or owner is itself potentially patentable and a second patent application might then be filed. Otherwise, the patent applicant or owner can keep the details of the improved method a secret as ‘undisclosed information’. The protection of undisclosed information is an independent type of intellectual property right. It rests on the foundation of the earlier Paris Convention (1883, as last amended in 1979). Art. 10 bis of the Paris Convention provides protection against acts of ‘unfair competition’, including any “…act of competition contrary to honest practices in industrial or commercial matters…”. Art. 39 TRIPS extends the ambit of this protection beyond the two types of undisclosed information commonly recognised before the TRIPS Agreement (‘trade secrets’ and ‘know-how’) to cover another, newly recognised type (‘test data’).
2.3.2. Trade secrets and know-how.
Trade secrets have long been recognised as specific bodies of undisclosed information with commercial value. A particular formula, for example, to make a soft drink, may be kept as a trade secret. Know-how is perhaps a less well-defined idea, understood as a broader body of undisclosed information which, taken in aggregate, is also commercially valuable. It can range from trade secrets down to, for example, technical designs and specifications, instruction manuals, process controls and monitoring, quality control procedures, technical training, working practices and other elements which, although individually not necessarily ‘groundbreaking’, might together nevertheless be difficult for a third party to reproduce.
Art. 39.2 TRIPS requires that:
“Natural and legal persons shall have the possibility of preventing information lawfully within their control from being disclosed to, acquired by, or used by others without their consent in a manner contrary to honest commercial practices (footnote) so long as such information:
(a) is secret in the sense that it is not, as a body or in the precise configuration and assembly of its components, generally known among or readily accessible to persons within the circles that normally deal with the kind of information in question;
(b) has commercial value because it is secret; and
(c) has been subject to reasonable steps under the circumstances, by the person lawfully in control of the information, to keep it secret.”
The footnote provides that ‘a manner contrary to honest commercial practices’ includes, for example, breach of contract, breach of confidence and inducement to breach.
The broader term ‘know-how’ can reasonably be understood to include the more narrow term ‘trade secret’ but not vice versa. For the sake of brevity this paper will therefore use know-how as a catch-all term for both. However, it is only know-how which exhibits these characteristics (a) – (c) which will be protected.
Such ‘know-how’ protection is favoured by intellectual property owners as it is independent of patent protection and will therefore continue even if the associated patent protecting the underlying product expires or is revoked. This independence has important consequences. Consider an example where a pharmaceutical company owns both a patent, which discloses a poor but sufficient method for making the invention, and know-how, which permits the effective and commercially viable production of the invention. Art. 31 TRIPS permits compulsory patent licences to be granted, as confirmed by the Doha Declaration on TRIPS and Public Health. Even if a third party obtains a compulsory patent licence, without access to the know-how they will not be able to produce the invention in an efficient and commercially viable way. Practically speaking, the exploitation of the compulsory patent licence would therefore be frustrated. Even if, for example, an employee of the owner of the patent and know-how believed it unconscionable not to permit the third party to make and sell the product under a compulsory licence lawfully granted on public health grounds, they would be restrained from disclosing the know-how through the threat of legal actions against them for breach of contract and / or breach of confidence.
This relationship between patents and know-how is, of course, well known in intellectual property circles:
“A very striking case about the importance of proprietary know-how comes from Brazil. Brazilian officials learned a quick and startling lesson when they decided, some years ago, to translate important patents that issued in developed countries into Portuguese for the benefit of Brazilian industry. They believed that this was all that was necessary to enable their industries to practice these foreign inventions without paying royalties for licenses. Needless to say, without access to the necessary know-how, this scheme was an utter failure.” (Jorda 2007)
and, therefore, in terms of advice:
“If an invention has been fully described so as to enable a person skilled in the art to make and use it, and if the best mode for carrying out the invention, if available, has been disclosed (as is required in a patent application), all associated or collateral know-how not divulged can, and should, be retained as a trade secret. All of the massive R&D data—including data pertaining to better modes developed after filing, whether or not inventive—should also be maintained as trade secrets, if the data is not disclosed in subsequent applications. Complementary patenting and padlocking is tantamount to having the best of both worlds, especially when technologies are complex and consist of many patentable inventions and volumes of associated know-how.” (Jorda 2007)
‘Having the best of both worlds’ does sound rather similar to ‘having their cake and eating it too’ does it not?
One important feature of the protection provided under Art. 39.2 TRIPS is that it prevents a third party from improperly acquiring or using know-how generated by another but it does not prevent that third party from trying to re-create that (or equivalent) know-how independently. A lesser degree of protection is therefore provided vis-à-vis patent rights which prevent a third party from, for example, making and selling an invention even if they have independently generated itself.
2.3.3. Test data.
Test data is data which has been generated in pre-clinical and clinical trials (and in other tests) which has to be submitted to regulatory authorities in order to demonstrate that the corresponding medical product meets the necessary efficacy, safety and quality requirements such that it can obtain marketing approval.
Art. 39.3 TRIPS requires that:
“Members, when requiring, as a condition of approving the marketing of pharmaceutical or of agricultural chemical products which utilize new chemical entities, the submission of undisclosed test or other data, the origination of which involves a considerable effort, shall protect such data against unfair commercial use. In addition, Members shall protect such data against disclosure, except where necessary to protect the public, or unless steps are taken to ensure that the data are protected against unfair commercial use.”
A number of different TRIPS-compliant models exist for the implementation of this obligation. Consider that a pharmaceutical company generates the necessary test data in order to obtain marketing approval. One model permits third parties to obtain marketing approval by relying on that test data and demonstrating that their product is equivalent. It would be unethical for the third party to have to repeat (unnecessary) clinical trials. It is important to note that this model does not require the test data to be disclosed to the third party but remains in the hands of the regulatory authorities. Such reliance could come with an obligation to compensate the owner of the test data (‘data compensation’) to offset the cost of undertaking the pre-clinical and clinical trials (and other tests). Some Members, especially High Income Country (HIC) Members, tend to favour another model, which instead forbids third parties from obtaining marketing approval by relying on that test data for a defined period of time. Although not required by the TRIPS Agreement (‘TRIPS-plus’), this model effectively creates a new intellectual property right: ‘data exclusivity’.
Data exclusivity is favoured by intellectual property owners in the pharmaceutical field as it is (again) independent of patent protection and will therefore continue for the defined period of time even if the patent protecting the underlying product expires or is revoked. This independence (again) has important consequences in that even if a third party obtains a compulsory patent licence, without being able to rely on the test data they won’t be able to obtain regulatory approval. Practically speaking, the exploitation of the compulsory patent licence would (again) be frustrated. If Members do implement the data exclusivity model, they should therefore provide for a corresponding public health waiver (c.f. ‘where necessary to protect the public’), for example, to permit regulatory approval to be obtained for a product produced by a third party under a compulsory licence. (See here for an ML&P briefing document on data exclusivity and waivers.)
3. The know-how gap problem.
3.1 The ‘intellectual property stack’.
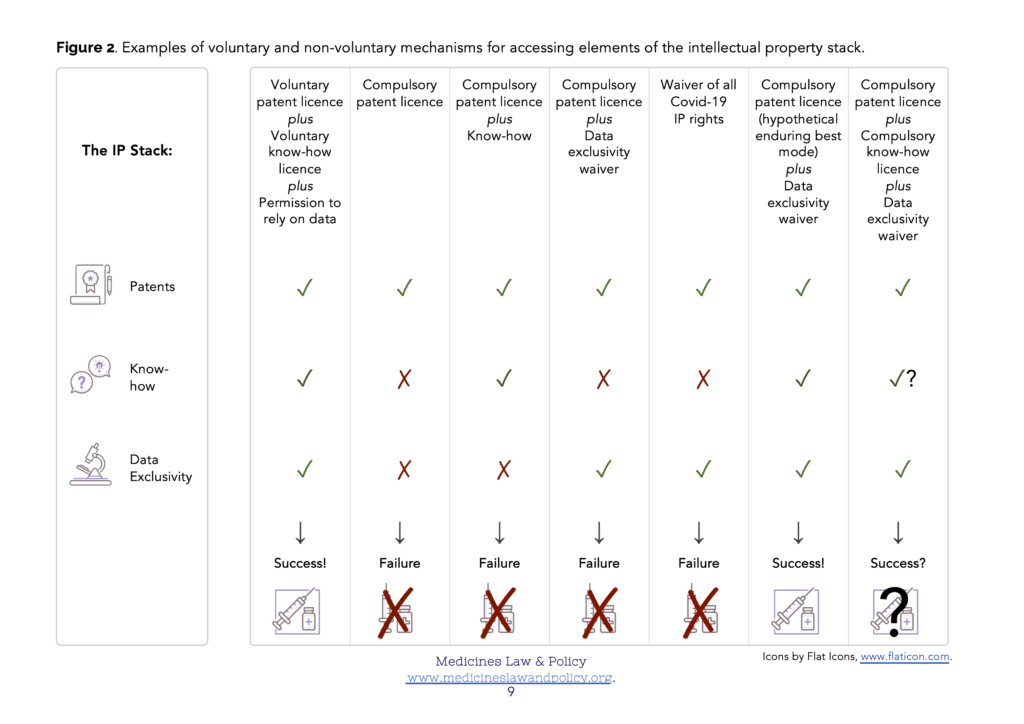
In many cases the information that is disclosed by a patent publication– a ‘sufficient’ description of how to carry out the invention or even the ‘best’ description known to the inventor at the patent filing (priority) date – will be inadequate to permit its effective and commercially viable production (Figure 1). This is especially likely to be true regarding some of the more technologically sophisticated Covid-19 related products, such as monoclonal antibody (mAb) therapies and mRNA-based vaccines. In order to enable commercially viable production (at scale), supplementary know-how will almost certainly be needed. In addition, though, reliance on test data will also be needed in order to obtain regulatory approval for the product eventually produced, and that may be forbidden if a data exclusivity implementation of Art. 39.3 TRIPS has been chosen. In those Members where they occur, the three rights – patents, know-how and data exclusivity – therefore form an ‘intellectual property stack’ (Figure 2). In order to make and supply the product, simultaneous access to all three elements of the stack will be required.
3.2 Voluntary access to the ‘intellectual property stack’.
In order to urgently scale up production of Covid-19 related products, the optimal mechanism will therefore likely be voluntary licensing and technology transfer. In this case, the owner of the ‘intellectual property stack’ will voluntarily provide appropriate third parties with a voluntary patent licence, the corresponding know-how and permission to rely on their test data (Figure 2, column 1). This could be done on a case-by-case basis, for example, the voluntary licensing programs such as that undertaken by Oxford University / AstraZeneca with their partners (see, for example, here).
Alternatively, voluntary licensing could be done in a more systematic way via the WHO-hosted Covid-19 Technology Access Pool (C-TAP) (and the Medicines Patent Pool (MPP)). In fact, there are two different C-TAP related mechanisms that could be considered. Firstly, a particular package of (‘foreground’) know-how for a particular product could be transferred via C-TAP. It is important, though, that partners have sufficient technical capability to be able to ‘absorb’ and use that know-how. Secondly, then, a range of packages of more general (‘background’) know-how could also be transferred via C-TAP, depending on the technical capability of the partner in question, to bring them all up to a level where they could absorb and use the particular (‘foreground’) packages of know-how.
3.3. Non-voluntary access to the ‘intellectual property stack’?
However, it is reasonable to observe that progress to date on voluntary measures has varied between patchy and deeply disappointing. In order to urgently scale up production of Covid-19 related products, Members are therefore entitled to consider non-voluntary options for accessing the ‘intellectual property stack’.
There is a clear danger that compulsory patent licences may be frustrated through lack of access to the know-how (Figure 2, columns 2 and 4) or test data (data exclusivity) elements (Figure 2, columns 2 and 3) of the stack. The relevant test data will at least have been provided to the regulatory authorities so long as regulatory approval is sought (an additional problem, not discussed here, will occur in a Member where regulatory approval is not sought and the test data has not therefore been provided). If a Member has chosen to provide for data exclusivity, then access can perhaps at least be provided via a corresponding waiver. Access to the know-how element is more challenging for a government to achieve as it is kept secret in the hands of its owner. The presently discussed proposal for a multilaterally agreed waiver of the need to enforce TRIPS obligations for Covid-19 related intellectual property rights will not solve this problem either. Even if agreed, such a waiver could not force the disclosure of know-how by its owners (Figure 2, column 5).
In practice, this danger is heightened by the fact that different elements of the stack are likely to be located in different Members. Even if a Member has granted a compulsory patent licence and granted a data exclusivity waiver, the know-how needed may be owned by a firm in another Member. Relevant know-how might be aggregated by a multinational pharmaceutical company from partners in several Members. It was recently reported, for example, that the scaling up of the Pfizer-BioNTech vaccine relies in part on know-how owned by Polymun, a small lipid nanoparticle manufacturer in Austria (“A key ingredient in what could be the first U.S.-approved Covid-19 vaccine comes from a family-owned company with 90 employees in the Austrian countryside, underscoring the fragility of the potential treatment’s supply chain.” (Wall Street Journal, 6th November 2020)). In order to help scale up production, Polymun have now agreed to transfer some of their know-how to Pfizer’s manufacturing facilities in Europe and the United States. It is easy to see how the effective exploitation of a corresponding compulsory patent license in, for example, a Low and Middle Income Country (LMIC) Member, might be difficult in such a case.
Unfortunately, even this does not exhaust all the ways in which the exploitation of a compulsory patent licence might be frustrated. Although not shown, the ‘stack’ can easily be extended to include a fourth ‘manufacturing capacity’ element. Even a compulsory patent licence granted by a Member with hypothetical access to the patent, know-how and test data elements of the ‘intellectual property / business stack’ could be frustrated if the necessary (end-to-end) manufacturing capacity element cannot be accessed, for example, if an essential element of the manufacturing process has been outsourced to another Member. (We have previously discussed the problem of the HIC opt-out to the Art. 31bis system in the context of Active Pharmaceutical Ingredient (API) production being outsourced to China and India).
3.4 Thinking about non-voluntary bridging of the ‘know-how gap’ problem
Bearing in mind that it is the know-how element of the stack which will likely often represent the most difficult problem to address in non-voluntary cases, how might this ‘know-how gap’ be bridged?
LMIC Members have long been advised to adopt a ‘best mode’ requirement under Art. 29.1 TRIPS. It is also sensible for them to think hard, for example, about how they should most appropriately define ‘person skilled in the art’. However, as illustrated in Figure 1, this may only mitigate the problem to a small extent. It is perhaps helpful to begin by framing a hypothetical solution to the problem with which to compare existing or proposed solutions.
Imagine how different the situation would be with an enduring best mode requirement: instead of just having to pay an annual renewal fee, an updated disclosure of the best mode of carrying out the invention known to the patent owner would have to be submitted as a free-standing annex each year in order to keep their granted patent in force. If a pharmaceutical firm (patent owner) had devised an improved method of carrying out their invention which permitted effective and commercially viable production (at scale), they would therefore have to disclose it at the next available (annual) opportunity if they wished to continue to benefit from the exclusive rights conferred by their patent. Figure 1 figuratively illustrates such a mechanism (right hand column). Figure 2, column 6 figuratively illustrates the outcome. Bearing in mind the TRIPS Agreement states its objective as follows (Art. 7 TRIPS):
“The protection and enforcement of intellectual property rights should contribute to the promotion of technological innovation and to the transfer and dissemination of technology, to the mutual advantage of producers and users of technological knowledge and in a manner conducive to social and economic welfare, and to a balance of rights and obligations.” (underlining added).
might such a maintenance requirement not seem reasonable in terms of the better balance this revised patent bargain would strike? From a public perspective, it would markedly reduce the likelihood that a compulsory patent licence would be frustrated since the corresponding know-how would necessarily be available. It would support the human right to health (c.f United Nations Committee on Economic, Social and Cultural Rights, General Comment No. 14 (2000)) and the human right to benefit from scientific progress (c.f United Nations Committee on Economic, Social and Cultural Rights, General Comment No. 25 (2020)).
Of course, patent owners would likely argue that this would be too heavy a burden for them to endure – they would prefer to stick with the ‘one-off’ bargain struck at the filing date. It could easily be argued, though, that the imposition of exclusive patent rights, with potentially life and death consequences in the pharmaceutical / medical field, is often too heavy a burden for the public to endure too. Other patent owners might argue that it would cause them not to undertake more development of the invention than the bare minimum required for the patent, but then there might be no commercial product at all, or that it would cause them to rely on know-how (trade secret) protection instead of patents, but then there would be no exclusive patent rights to deal with. Notwithstanding the long and complex history of the intellectual property system to date, there is still room for substantial debate about where the optimal balance between innovation, dissemination and access lies.
Against the background of such a hypothetical solution, though, how else might the know-how gap problem be addressed in a non-voluntary way in the urgent circumstances of the Covid-19 pandemic? Perhaps the most straightforward suggestion is that of a compulsory know-how licence, especially if considered as an ancillary order to accompany a compulsory patent licence. It is arguable that such a suggestion is TRIPS-compliant (Wang 2014). Helpful parallels can be made with equivalent mechanisms in Antitrust law. However, there still remains, for example, the practical problem of one Member enforcing such a compulsory know-how licence against a firm in another Member (Figure 2, column 7). A future note will examine this suggestion further. Another suggestion might involve a collaborative R&D program to reverse engineer, recreate and share the necessary know-how independently of its original owner. Even if this were possible, though, any such program would likely represent a diversion of important resources at a critical time.
3.5 The immediate future.
Although it is vital that Members are able to make use of effective non-voluntary measures, the most certain and quick way to solve the know-how gap problem, especially for the more technologically sophisticated Covid-19 related products, will likely be through voluntary licensing and technology transfer. Given the huge sums of public money being committed to underwrite the development of Covid-19 related products, it is entirely appropriate for Members to use the leverage that funding provides to encourage intellectual property rights holders’ to engage in voluntary licensing and technology transfer, especially through platforms such as C-TAP (and the MPP). It is to be hoped that such encouragement soon begins to bear fruit.
References
Carlson, D.L., Przychodzen, K. and Scamborava, P., 2005, Patent Linchpin for the 21st Century – Best Mode Revisited, 87 J. Pat. & Trademark Off. Soc’y 89. Available, via hosting at www.wiggin.com, at: https://www.wiggin.com/wp-content/uploads/2019/09/best-mode-article-2005-carlson.pdf
Jorda, K.F., 2007, Trade Secrets and Trade-Secret Licensing, Chapter 11.5 in MIHR / PIPRA IP Handbook of Best Practices, available at: http://www.iphandbook.org/handbook/ch11/p05/
Wang, L.R., 2014, Ancillary Orders of Compulsory Licensing and Their Compatability with the TRIPS Agreement, 18 Marq. Intellectual Property L. Rev. 87. Available at: http://scholarship.law.marquette.edu/iplr/vol18/iss1/3
Acknowledgements
This briefing paper has been prepared by Christopher Garrison with the assistance of other members of Medicines Law & Policy: Dr Ellen ’t Hoen, Pascale Boulet, Dr Katrina Perehudoff, and Kaitlin Mara. Any questions or requests for further information should be directed to info@medicineslawandpolicy.net.
Christopher Garrison, MA LLM MA PhD, is a legal advisor with over 20 years of experience on intellectual property issues.